The metal in potassium hydroxide is heavier than the metal in sodium hydroxide. Molecular weight of potassium hydroxide is 5611 g mol 1 Molecular weight of sodium hydroxide is 39.
Sodium Hydroxide Vs Potassium Hydroxide Coloring Pages - If you're searching for video and picture information linked to the key word you've come to pay a visit to the ideal blog. Our website gives you hints for seeing the highest quality video and picture content, search and find more informative video content and graphics that fit your interests. comprises one of thousands of movie collections from various sources, especially Youtube, therefore we recommend this movie for you to see. It is also possible to bring about supporting this website by sharing videos and graphics that you like on this blog on your social networking accounts such as Facebook and Instagram or tell your closest friends share your experiences concerning the simplicity of access to downloads and the information you get on this website. This blog is for them to stop by this site.
Effect Of Sodium Hydroxide And Potassium Hydroxide In Methanol On The Download Table
An estimated 700000 to 800000 tonnes were produced in 2005.
Sodium hydroxide vs potassium hydroxide coloring pages. Potassium has an atomic weight of 3910 and sodium has an atomic weight of 2299. The next highest concentration of use for sodium hydroxide in a leave-on product is 69 in a face or neck product. Potassium hydroxide soaps are softer and more easily dissolved in water than sodium hydroxide soaps.
Must have a pH below 127 when used as a pH adjuster in depilatories. Calcium hydroxide CaOH2 which will be formed is relatively insoluble it has a Solubility product Ksp of only 55106 which equals a solubility in water of only 17. Well remember previously I said about the bonds.
I have to disagree with professor Samuel Mahtab I came to another conclusion. KOH is noteworthy as the precursor to most soft and liquid soaps as well as numerous potassium. Sodium hydroxide and potassium hydroxide may not exceed 5 in nail cuticle solvents.
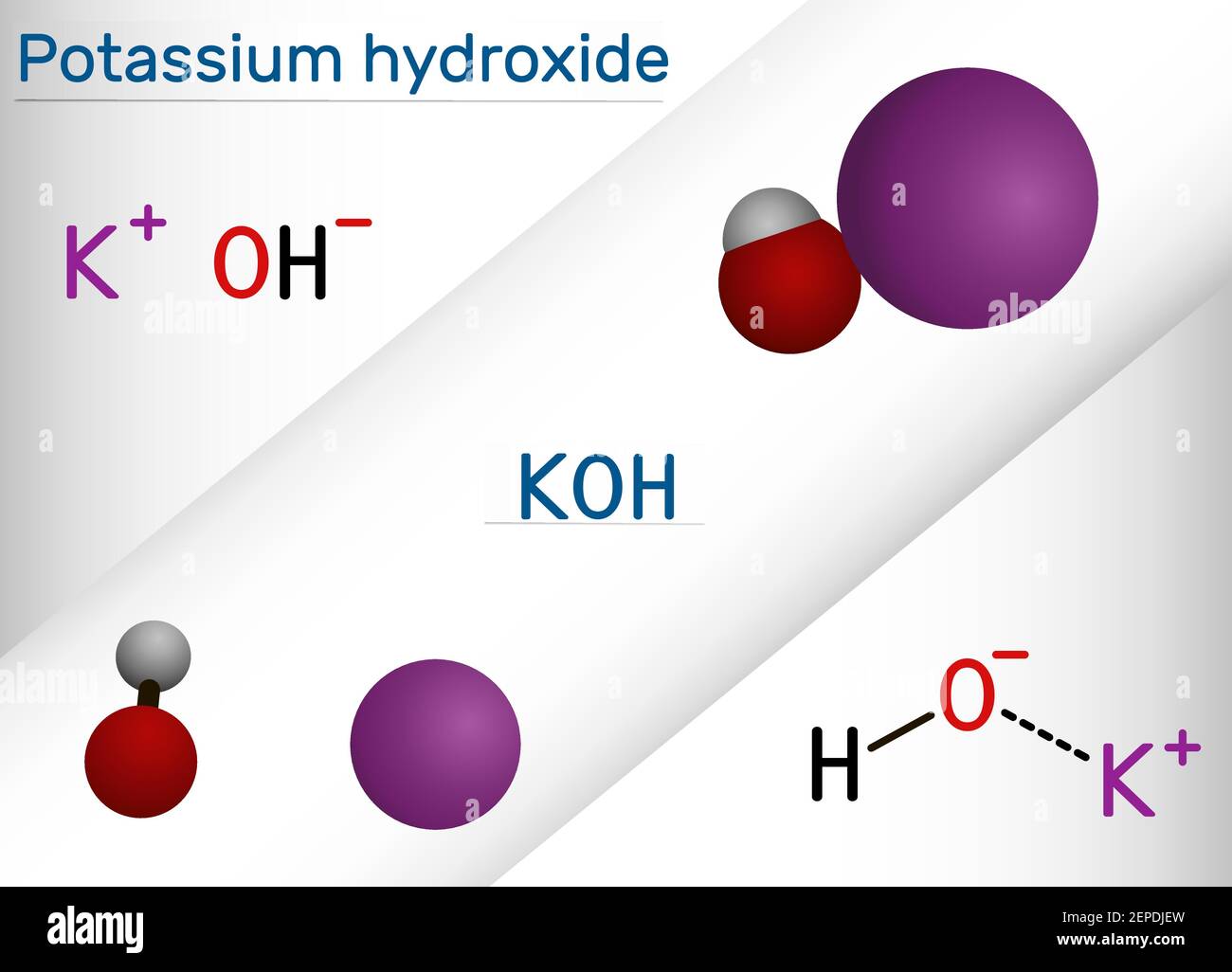
Potassium hydroxide is an inorganic compound with the formula KOH and is commonly called caustic potash. Molecular model of the caustic metallic base sodium hydroxide NaOH mostly used as a strong chemical base in the production of pulp and paper textiles drinking water soaps and detergents. Boric acidpotassium chloridesodium hydroxide coloured.
Since they are also more soluble the oils can be rinsed away easier especially when using. Well sodium hydroxide will react with the oils causing the molecules to be denser and tighter as compared to potassium hydroxide therefore its more suitable for solid soap. Blue traceable to SRM from NIST and PTB pH 900 20C Certipur Pricing.
Potassium hydroxide and sodium hydroxide are both caustic bases formed from an alkali metal ionically bound to a hydroxide group. Sodium hydroxide and potassium hydroxide are not interchangeable in either the proportions required or the properties produced in making soaps. Eye exposure to concentrated sodium hydroxide or potassium hydroxide solutions can cause severe eye damage and possibly blindness.
Contact with even dilute solutions will also cause skin irritation and injury the severity of which will depend on the duration of contact. Along with sodium hydroxide KOH is a prototypical strong base. 109409 boric acidpotassium chloridesodium hydroxidetraceable to SRM from NIST and PTB pH 1000 25C Certipur Pricing.
Potassium hydroxide is used up to 7 in a leave-on body and hand product. Atoms are represented as spheres and are colour-coded. Commercial sources are marketed in several forms including pellets flakes sticks lumps 40 and powders.
This makes potassium hydroxide a great choice for soaps that need to remove caked-on oil. 38 Potassium hydroxide is a white to slightly yellowish solid that is highly deliquescent prone to 39 liquification. What is the difference between Sodium Hydroxide and Potassium Hydroxide.
When dissolved in water or alcohol potassium hydroxide. The main difference between Potassium hydroxide and Sodium Hydroxide is that Potassium hydroxide has a potassium cation and a -OH anion whereas Sodium Hydroxide has a sodium cation and OH anion. Potassium hydroxide is slightly smaller than sodium hydroxide which means it cuts through oil molecules faster than sodium hydroxide.
9971 g mol 1. Potassium has an atomic weight of 3910 and has 19 protons. This makes the metal in potassium hydroxide heavier than the metal in sodium hydroxide.
Although we use the terms lye and caustic soda interchangeably they are slightly different from each other because lye is a general term while caustic soda is a specific name. Yes and no. It has many industrial and niche applications most of which exploit its caustic nature and its reactivity toward acids.
These hydroxides are strong bases and are very corrosive. They are the hydroxides of two consecutive members of group I metals. Hydrogen white oxygen red and sodium violet.
Sodium hydroxide is used at up to 10 in an other skin care preparation which may or may not be a leave-on product. In ton quantities potassium hydroxide is about three times more expensive than sodium hydroxide¹ At the molecular level potassium hydroxide is also slightly smaller than sodium hydroxide. Potassium has 19 protons and sodium.
Lye in the form of both sodium hydroxide and potassium hydroxide is used in making soap. Leave-on products deodorants. Sodium Na and Potassium K.
Potassium hydroxide is somewhat more corrosive than sodium hydroxide. Therefore it can penetrate oil molecules faster than sodium hydroxide thus breaking the oils hold on surfaces quicker. The metal in sodium hydroxide is lighter than the metal in potassium hydroxide.
The key difference between lye and caustic soda is that the term lye may refer to either sodium hydroxide or potassium hydroxide whereas the term caustic soda refers only to sodium hydroxide. Sodium has an atomic weight of 2299 and has only 11 protons. 2 for general use and 45 in professional use of hair.
And must have pH below 11 in other uses. Potassium hydroxide on the other hand is more soluble in water so its more suitable for liquid soap. This weight difference reflects the difference in the number of protons in each metal.
Potassium Hydroxide High Resolution Stock Photography And Images Alamy
Effect Of Sodium Hydroxide And Potassium Hydroxide In Methanol On The Download Table
Potassium Hydroxide High Resolution Stock Photography And Images Alamy
28 Sodium Hydroxide Illustrations Clip Art Istock
Why Alkalies Like Sodium Hydroxide And Potassium Hydroxide Should Not Be Left Exposed To Air
568 Sodium Hydroxide Photos Free Royalty Free Stock Photos From Dreamstime
568 Sodium Hydroxide Photos Free Royalty Free Stock Photos From Dreamstime
568 Sodium Hydroxide Photos Free Royalty Free Stock Photos From Dreamstime
Effect Of Sodium Hydroxide And Potassium Hydroxide In Methanol On The Download Table
Related Posts
- Pentagon Coloring Pages Do-a-dot q-tip-painting 8 Pentagon Worksheets. The Pentagon Building in Washington DC is the headquarters of the United States Armed Forces and the ...
- Making An Abc Book For Your Baby Coloring Pages Cut a horizontal line across the center of each page. Then we had each person draw or color a page.Making An Abc Book For Your Baby Coloring Pages - ...
- Minion Avengers Coloring Pages Books colouring avengers game jumbo coloring book pages marvel comic. Nov 21 2018 - Explore Bryantjessis board Minion coloring pages on Pinterest.Mi ...
- Man Steel Logo S Coloring Pages Download and print these Man Of Steel coloring pages for free. Man of steel Results 5520 - 5521 of.Man Steel Logo S Coloring Pages - If you're ...
- Journalist Coloring Pages Also look at our large collection of cartoon coloring pages for preschool kindergarten and grade school children. Featuring Olympic sports for summe ...
- Cyrus Coloring Pages Go to that enlarge coloring page and press CTRL P on your keyboard to print your coloring page. Joseph Coloring Pages - Best Coloring Pa.Cyrus Color ...
- Minion Png Coloring Pages As a rambunctious bunch of simple-minded homunculi Minions are a similar size and shape but have unique features to tell them apart such as height n ...
